
Thank you for your interest in Biatain® Silicone
Please fill out the form below, specifying your contact preference, and a representative will follow-up with you to discuss next steps. You must be an active healthcare professional within the United States.
Managing Infection
New White Paper
Managing Wound Infections & Biofilm
A new White Paper offers a simplified yet practical summary for the management of wound infections and biofilms, based on a review of published consensus, guidance and best practice standards.
Watch the videos below for an introduction to the new White Paper.
The Problem
Exudate pooling could increase the risk of bacteria growth and infection, which can delay wound healing and may lead to severe complications.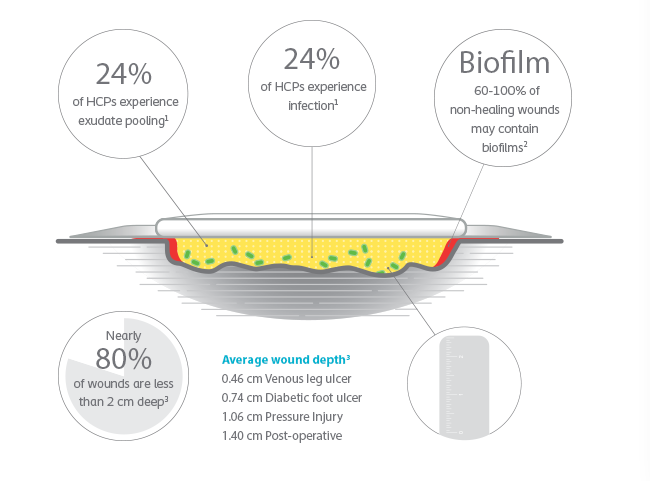
The Solution
Biatain® Silicone with 3DFit Technology reduces the risk of gaps and pooling.
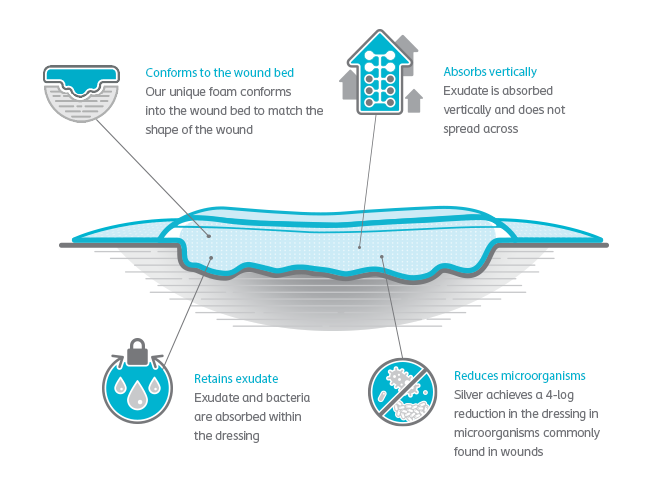
Does your current dressing match the shape of the wound?
In a large case study series, Biatain Silicone foam dressings conformed exactly to the shape and depth of 104 different wounds, creating an intimate fit, and reducing the risk of pooling and bacteria growth in the wound.4
Biatain Silicone Ag absorbs bacteria and exudate, including thicker, more viscous exudate, even under compression.
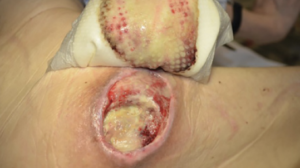
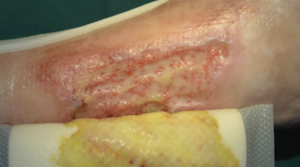
Biatain Silicone Ag sustains antimicrobial activity for up to 7 days, and disrupts, reduces and prevents regrowth of bacterial aggregates.
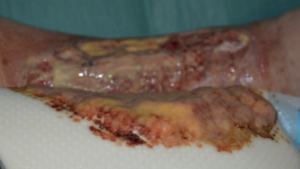
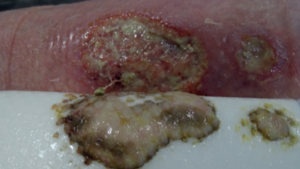
Significant reduction of bioburden in the wound bed
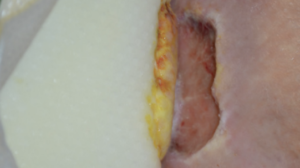
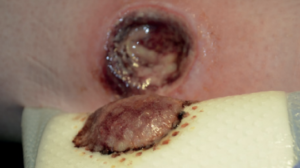
From soft tissue punch biopsies on 19 patients, Biatain Silicone Ag was shown to achieve a significant reduction of bioburden in pathogens such as S. aureus and P. aeruginosa. The case below is a 67-year-old patient with an infected diabetic foot ulcer that had been present for 8 weeks prior to treatment with Biatain Silicone Ag5
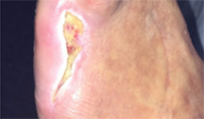
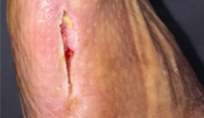
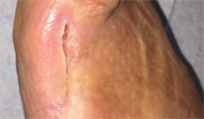
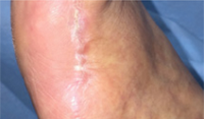
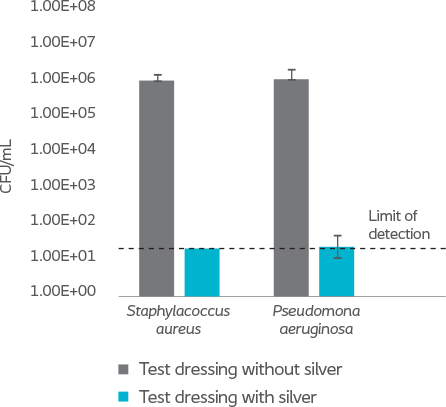
The results of an in-vitro test demonstrated that Biatain Silicone Ag reduced all tested microorganisms, including antibiotic resistant strains, by more than log 4 (10,000-fold)6.
In a separate in-vitro test, a wound model from the University of Copenhagen7 was used to grow bacterial aggregates, including P. aeruginosa and S. aureus., and Biatain Silicone Ag was demonstrated to disrupt, reduce and prevent regrowth of bacterial aggregates.8

References
1. Digital survey conducted by Coloplast among 1800 healthcare professionals, Spring 2018
2. World Union of Wound Healing Societies (WUWHS), Florence Congress, Position Document: Management of Biofilm, Wounds International 2016
3. Braunwarth et al., Wound depth and the need of a wound filler in chronic wounds, Wounds UK, 2017
4. Braunwarth et al., Conformability of a foam dressing – clinical experience based on 104 cases, Wounds UK, 2017
5. Lázaro-Martínez et al., Clinical and antimicrobial efficacy of a silver foam dressing with silicone adhesive in diabetic foot ulcers with mild infection, Journal of Lower Extremity Wounds, August 2019
6. Data on file, Determination of the antimicrobial effectiveness of Biatain Silicone Ag using a modified AATCC 100 test method, VV-0254113, January 2020
7. Crone et al., A novel in vitro wound biofilm model used to evaluate low frequency ultrasonic-assisted wound debridement, Journal of Wound Care, 2015
8. Christiansen et al., In vitro evaluation of a silver foam dressing with and without silicone adhesive against biofilms and a broad range of microorganisms, EWMA 2018

